FreeStyle Libre 3 Sensors Voluntary Medical Device Correction in the U.S. Issued by Abbott
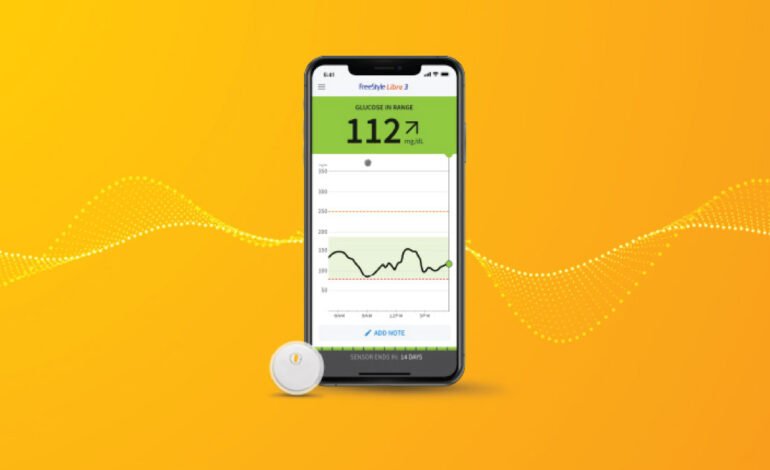
In response to possible erroneous glucose readings, Abbott has proactively provided a voluntary medical device repair for a restricted number of FreeStyle Libre 3 sensors in the United States, demonstrating its dedication to patient safety and product integrity.
Abbott’s Proactive Stance on FreeStyle Libre 3 Sensor Correction
Abbott has disclosed a voluntary medical device repair that would impact a small number of its FreeStyle Libre 3 sensors in the United States in a regulatory update. This action highlights Abbott’s commitment to patient safety and the integrity of its product line. It was taken in response to the discovery that some sensors may give erroneous glucose readings.
With its ability to provide patients with accurate and continuous glucose data, the FreeStyle Libre 3 system—which is well-known for its real-time glucose monitoring—has significantly improved the management of diabetes. The technology has become a popular option for people looking to have more control over their blood sugar levels because of its small size and easy to use interface. However, Abbott discovered that a small percentage of sensors might not satisfy the high accuracy requirements set by the company, prompting this voluntary correction.
Advantages of the FreeStyle Libre 3 Technology
Continuous Monitoring: Users can avoid frequent finger pricks by using the FreeStyle Libre 3’s round-the-clock glucose monitoring feature. With real-time data available to support better management and decision-making, this innovation has completely changed the way diabetes is cared for.
Compact Design: The sensor’s tiny size and light weight make it easy to wear discreetly, which promotes regular use and adherence to diabetic treatment regimens.
User-Friendly Interface: Users may quickly obtain their glucose data by syncing the gadget with smartphones in an easy and seamless manner. The user-friendly layout of the app facilitates the interpretation of glucose trends and patterns, hence improving patient empowerment and participation.
Excellent Accuracy: The FreeStyle Libre 3 system is renowned for its excellent accuracy, offering dependable glucose readings that support patients in maintaining ideal blood sugar levels, even in light of the most recent correction notice. By addressing a small fraction of sensors, the present fix makes sure that most users continue to receive accurate data.
Conclusion
Abbott’s voluntary rectification of a limited number of FreeStyle Libre 3 sensors illustrates the company’s steadfast commitment to patient safety and product quality. By resolving any mistakes in a proactive manner, Abbott assures that users of the FreeStyle Libre 3 system may continue to believe in the accuracy and dependability of their glucose monitoring devices. Future developments in technology are expected to reinforce the FreeStyle Libre 3’s standing as a pioneer in diabetes treatment.