IOPS Viewpoint Catheter Gains FDA Clearance, Transforming Endovascular Surgery Safety
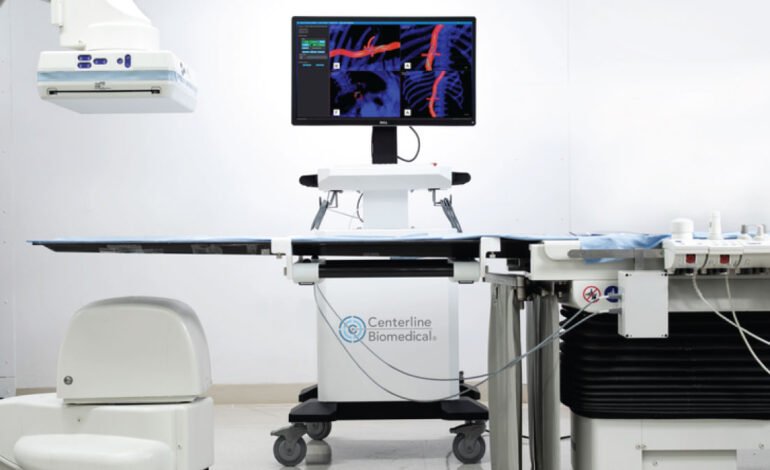
Centerline Biomedical has reached a major milestone with the FDA’s approval of its IOPS Viewpoint Catheter, a state-of-the-art device aimed at improving safety and accuracy in procedures that use images to guide medical interventions.
Pushing the Boundaries of Medical Device Innovation with IOPS Viewpoint Catheter
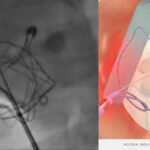
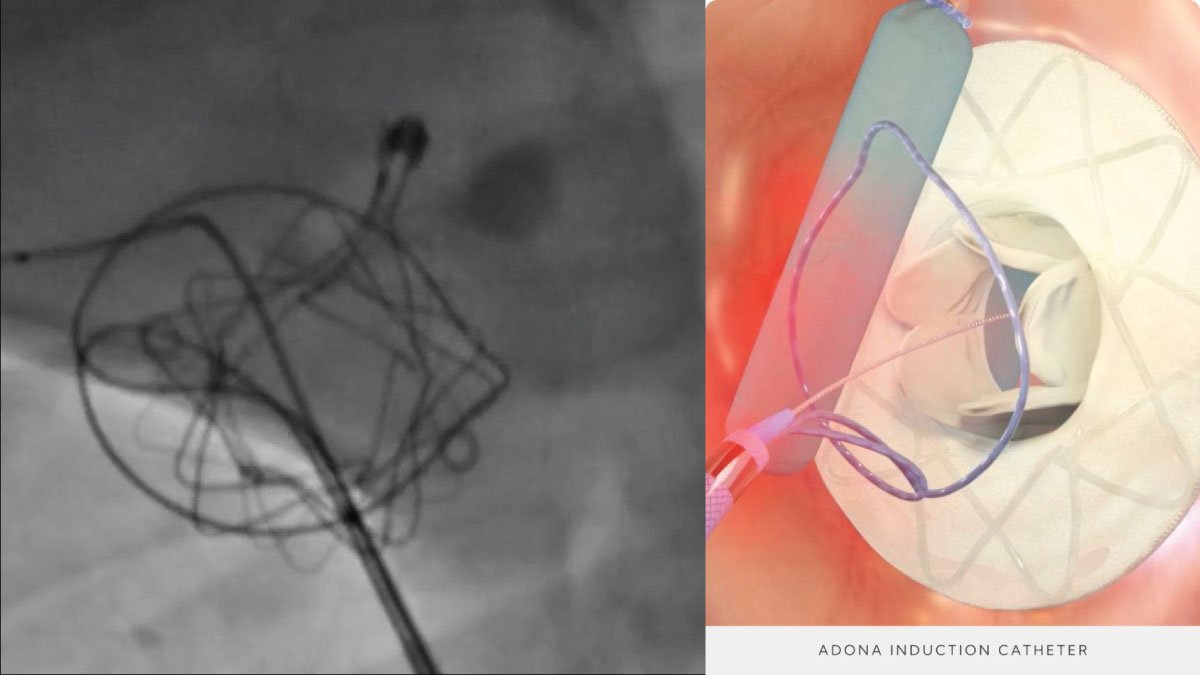
The recent approval of the IOPS by the FDA represents a significant breakthrough in the field of endovascular surgery. This cutting-edge technology is designed to lessen the dependence on potentially harmful X-ray radiation during procedures, providing a safer option for both patients and medical staff. The traditional approach exposes doctors to significant levels of radiation over time, which can lead to severe health issues. This tackles this problem by employing real-time, 3D navigation technology that reduces the need for X-ray imaging.
Possibilities and Advantages of IOPS Viewpoint Catheter
The IOPS Viewpoint Catheter is poised to transform a variety of medical procedures, especially those in vascular surgery where accuracy is crucial. By offering detailed 3D visualizations of blood vessels, the catheter enables surgeons to navigate with greater precision, thereby minimizing the chance of complications. This is particularly advantageous in complex surgeries like aneurysm repairs, stent placements, and other endovascular procedures where traditional imaging methods may not suffice.
Moreover, the technology is expected to shorten the duration of procedures, which could lead to better patient outcomes and lower healthcare expenses. The catheter’s ability to reduce radiation exposure is another key benefit, protecting the health of surgeons who frequently perform these procedures.
Future Developments in IOPS Viewpoint Catheter
The IOPS Viewpoint Catheter is a part of a larger movement towards minimizing radiation exposure in medical practices. As healthcare progresses towards more advanced and safer technologies, we anticipate further innovations that prioritize the health of both patients and medical professionals. The IOPS Viewpoint is likely to become a benchmark in the industry, influencing future advancements in medical devices that focus on reducing procedural risks while improving precision and effectiveness.
In the years ahead, the integration of real-time 3D navigation systems with other emerging technologies, such as artificial intelligence and machine learning, could further revolutionize the field of minimally invasive surgery. As these technologies continue to evolve, they are expected to expand into other medical areas, offering new opportunities for enhancing patient care and surgical results.
Conclusion
The FDA’s approval of the IOPS Viewpoint Catheter by Centerline Biomedical marks a significant advancement in the domain of endovascular surgery. By diminishing radiation exposure and enhancing surgical accuracy, this innovative device has the potential to redefine the standards of patient care. As the medical community continues to embrace these advancements, the future of safer, more efficient surgical techniques looks bright.