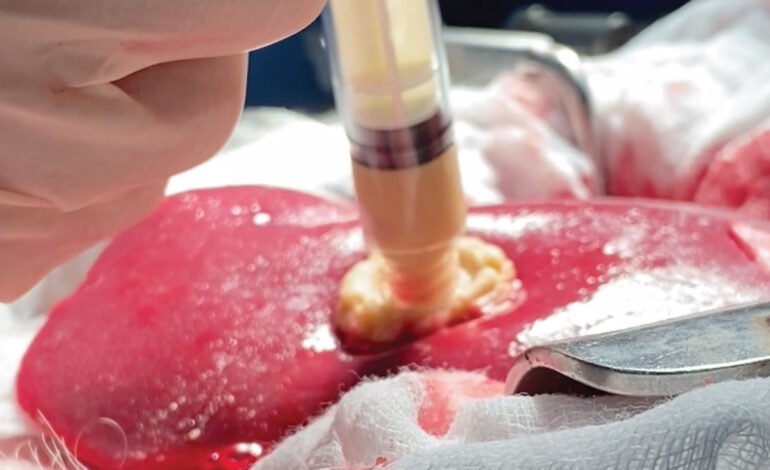
Cresilon TraumaGel FDA Clearance: New Gel Stops Severe Bleeding Instantly
Quick Read:
Cresilon TraumaGel gets FDA Clearance, an innovative hemostatic tool created to halt intense bleeding almost immediately. This technology offers fresh opportunities for quick control of bleeding in emergency situations and trauma treatment, meeting essential requirements in the medical device industry.
Cresilon TraumaGel gets FDA Clearance, Signaling a Major Breakthrough in Stopping Bleeding
The U.S. Food and Drug Administration (FDA) has given the green light to Cresilon’s TraumaGel, a groundbreaking hemostatic gel designed to quickly stop severe bleeding within minutes. This innovative solution promises to revolutionize emergency medical treatment by rapidly halting blood flow in critical situations, potentially saving countless lives. Cresilon’s cutting-edge technology employs a plant-based gel to form an immediate barrier over the wound, preventing additional blood loss.
Benefits of Cresilon’s TraumaGel Technology
The standout feature of TraumaGel’s technology is its efficiency in emergency scenarios, thanks to its simplicity and swift action. By generating a stable clot in mere seconds, it minimizes blood loss without the necessity for extra pressure or bandages. This aspect is particularly advantageous for serious injuries in remote or difficult-to-reach areas where immediate medical attention might not be available. Moreover, TraumaGel is derived from plant sources, eliminating the risk of allergic reactions or complications from synthetic materials. Its safety and compatibility with biological systems position it as an ideal choice for a variety of medical disciplines, from military use to high-impact situations, thereby expanding its potential for use and acceptance.
Uses in Medical Devices and Emergency Care
TraumaGel’s rapid action and ease of use open up a broad spectrum of applications across various medical devices and emergency settings. From its inclusion in basic first-aid kits to its integration into sophisticated trauma care systems, TraumaGel could become a standard component in both civilian and military medical responses. Its effectiveness is well-suited for pre-hospital and field trauma care, making it a top choice for emergency personnel. Furthermore, the gel’s unique composition allows for its incorporation into wearable devices or patches for continuous control of bleeding, offering a novel approach to patient care in severe injuries.
Future Prospects of Cresilon TraumaGel FDA Clearance and Expansion Potential
The FDA’s approval of TraumaGel is expected to spur further developments in hemostatic medical devices, with the creation of new formulations aimed at specialized trauma care. Its success could also inspire the development of more plant-based hemostatic solutions, especially in surgical procedures, wound care, and high-risk sports. The demand for fast-acting, safe solutions for controlling bleeding is likely to grow as more companies explore similar technologies for trauma care, potentially leading to its wider application in home healthcare, hospital environments, and more.
Conclusion
The FDA’s endorsement of Cresilon’s TraumaGel marks a significant milestone in the field of emergency and trauma care. Its capability to instantly stop severe bleeding addresses a critical need within the medical device sector and establishes a new benchmark in trauma response technology. With its broad applications and potential for future advancements, TraumaGel may transform the standards of emergency care, emphasizing rapid intervention and patient safety.